We are proud to announce that the InsightPFA study, led by Prof. Yumei Xue's team at Guangdong Provincial People’s Hospital, has been published in the Journal of the American College of Cardiology (JACC)—one of the most influential journals in cardiovascular medicine.
This publication marks a major milestone in cardiac electrophysiology as the world’s first randomized controlled trial (RCT) comparing nanosecond pulsed field ablation (nsPFA) with ablation index (AI)–guided radiofrequency ablation (RFA), addressing a critical evidence gap and advancing global understanding of pulsed-field ablation for AF.

Pulsed field ablation (PFA) has emerged as a promising non-thermal alternative for atrial fibrillation ablation. However, PFA systems under clinical development mostly use microsecond pulses, which are associated with significant skeletal muscle contractions and often require general anesthesia.
In contrast, nanosecond PFA has been shown in preclinical studies to markedly reduce muscle contraction while achieving effective myocardial electroporation. The LotosPFA™ catheter and InRythm™ system previously demonstrated strong performance in a porcine PVI model, yet clinical evidence in humans was lacking—leading to the launch of the InsightPFA trial.
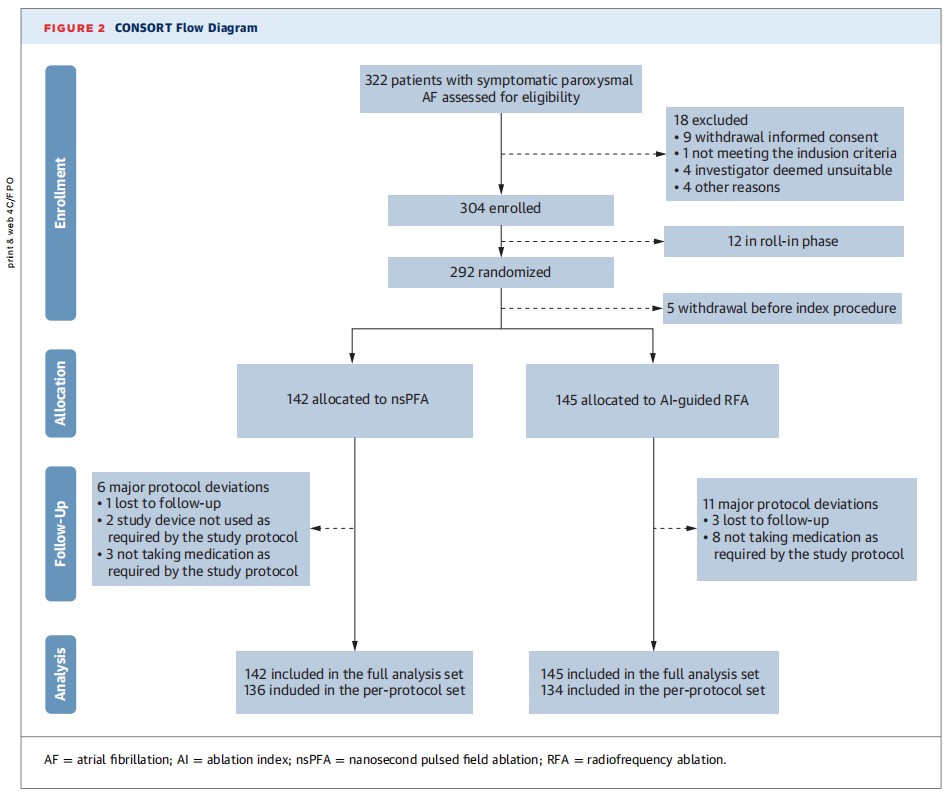
The InsightPFA trial (NCT06014996) was a prospective, multi-center, randomized, noninferiority clinical trial conducted across 13 centers with 27 operators. Eligible patients with symptomatic paroxysmal AF were randomized 1:1 to receive either nsPFA or AI-guided RFA for pulmonary vein isolation (PVI), with a standardized 12-month follow-up.
A total of 287 patients underwent ablation (142 nsPFA; 145 RFA), with well-balanced baseline characteristics across groups.
Using the LotosPFA™ catheter, operators delivered biphasic, nanosecond energy at 2100 V, applying four spindle-configuration applications and four lotus-configuration applications. Bidirectional PV block was confirmed after a 20-minute observation period or with 3D mapping.
A standard left atrial model was created, and ablation was performed using an AI target of 400–450 (anterior) and 300–350 (posterior). CTI ablation was added when typical flutter coexisted.
Both groups maintained ACT >300 seconds, and standardized anticoagulation was applied post-procedure.
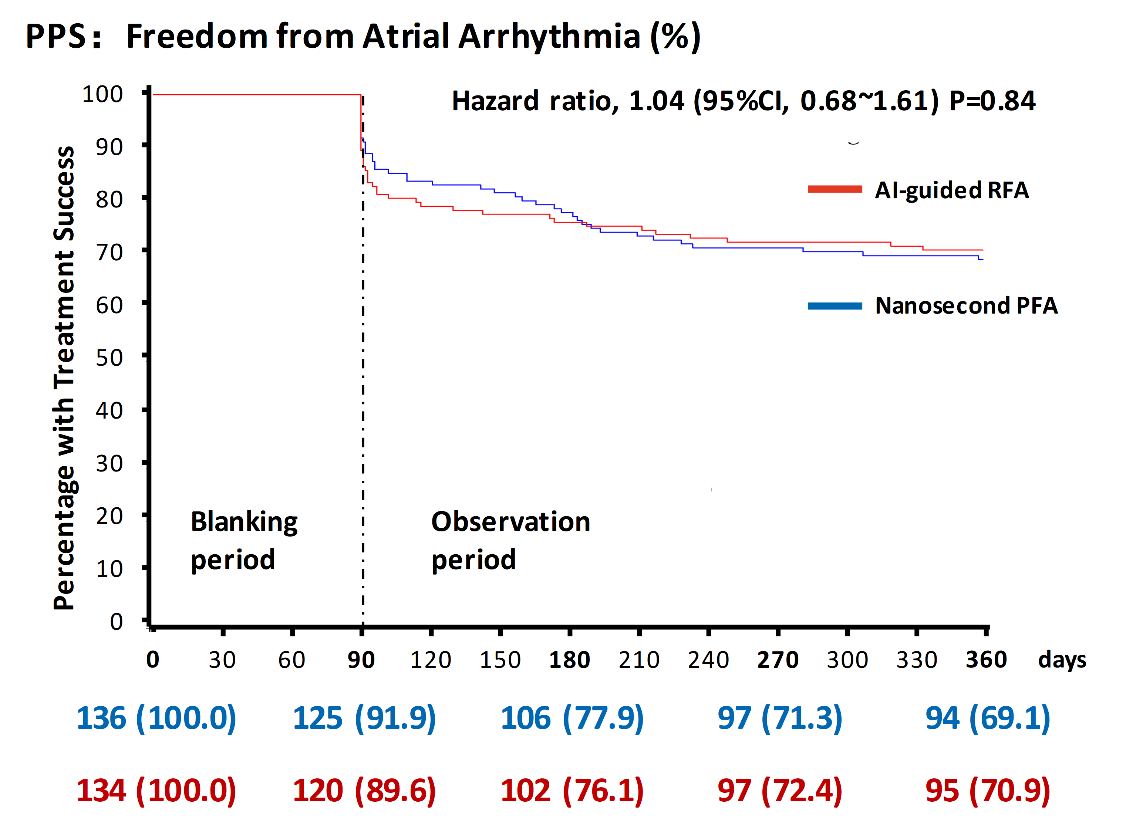
The study met its primary endpoint, demonstrating noninferior 12-month efficacy of nsPFA compared with AI-guided RFA.
In the per-protocol set:
This trial represents a key milestone in cardiac electrophysiology, providing the first randomized clinical evidence for nanosecond-pulse PFA in AF ablation.
nsPFA demonstrated noninferior efficacy, similar safety, and greater procedural efficiency versus AI-guided RFA, offering a promising new electroporation modality for pulmonary vein isolation. Its consistent feasibility under conscious sedation—with minimal muscle contraction—also suggests workflow and patient-comfort advantages. These findings add meaningful clinical evidence to the global evolution of pulsed field ablation technologies and support nsPFA’s role in next-generation AF ablation strategies.
Reference
2102, Tower A, Hongrongyuan North Station Center,
North Station Community, Minzhi, Longhua District,
518000 Shenzhen, PEOPLE'S REPUBLIC OF CHINA