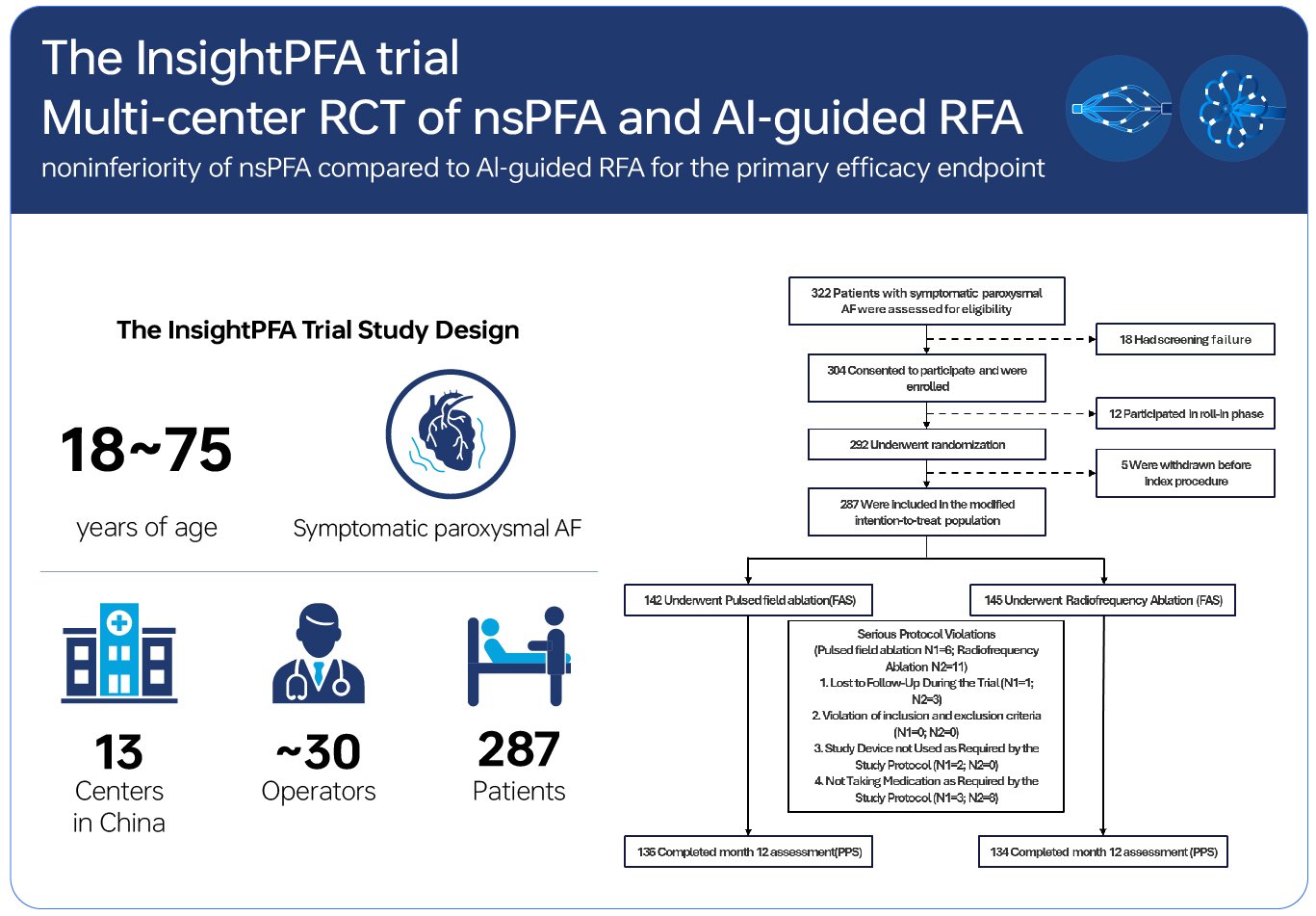
The InsightPFA trial, led by Insight Medtech (a subsidiary of Insight Lifetech Group), marks a milestone in cardiac electrophysiology as the world's first randomized controlled trial (RCT) of nanosecond pulsed field ablation (nsPFA) compared with pressure-sensing radiofrequency ablation (RFA).
As the highest level of evidence in clinical medicine, RCTs are regarded as the gold standard for validating new technologies. The results of InsightPFA were unveiled at the Late-Breaking Clinical Trials session at the ESC Congress 2025 in Madrid, highlighting its global scientific impact and clinical relevance.
 Late Breaking Clinical Trials Session at ESC Congress 2025
Late Breaking Clinical Trials Session at ESC Congress 2025
Designed for Real-World Impact
This study was led by Professor Xue Yumei from Guangdong Provincial People's Hospital as the Principal Investigator (PI) and jointly conducted by 13 authoritative centers across China. Adopting a prospective, randomized controlled design, the study systematically compared the safety and efficacy of nsPFA and RFA in the treatment of paroxysmal atrial fibrillation (PAF). A total of 292 patients with symptomatic paroxysmal atrial fibrillation were enrolled, with 146 patients in both the PFA group and the RFA group.
To ensure the scientific validity of efficacy assessment, the study strictly implemented a follow-up protocol, which included 24-hour Holter monitoring at 3, 6, and 12 months. Meanwhile, the extensive participation of multiple centers and operators made the study results more in line with real-world clinical practice, thereby increasing their reference value.
The results showed that statistical tests confirmed the achievement of the preset non-inferiority criteria, verifying that nsPFA achieved non-inferior long-term efficacy compared with AI-guided RFA in the treatment of paroxysmal atrial fibrillation.
Results That Reshape the Standard
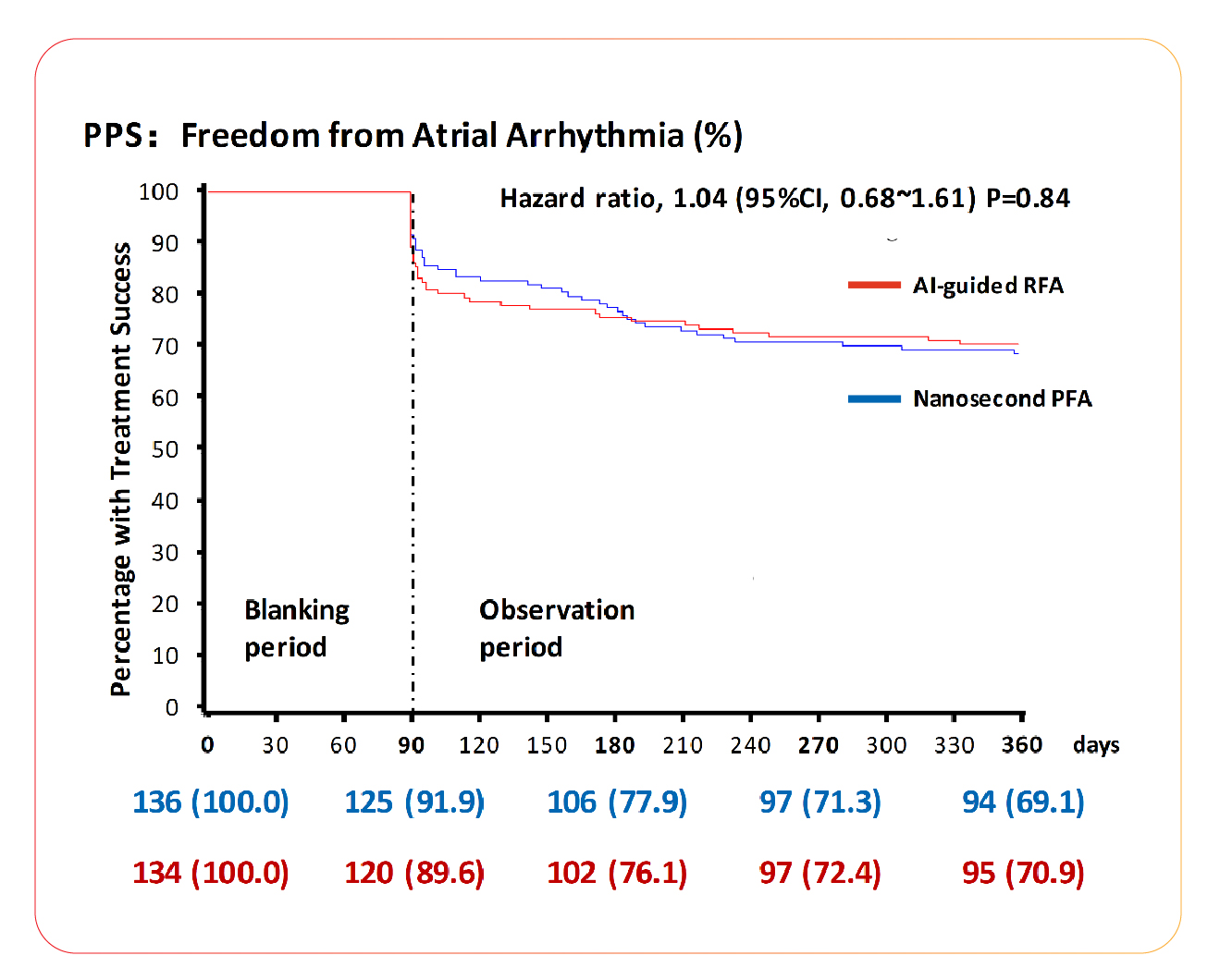
In the Per Protocol Set (PPS), the success rate was 69.1% in the nsPFA group and 70.9% in the AI-guided RFA group (HR 1.04, 95% CI [0.68-1.61]; P=0.84), demonstrating that nanosecond pulsed field ablation (nsPFA) exhibited comparable 12-month long-term efficacy to the well-established RFA.
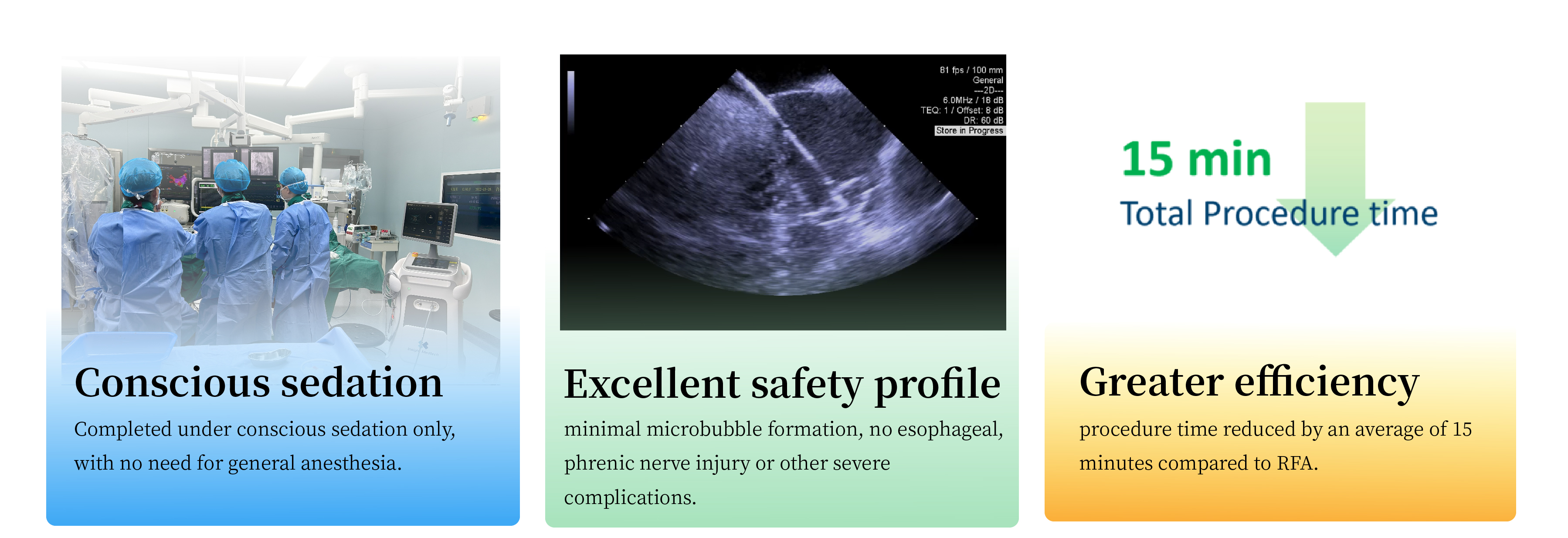
Unique advantages of the LotosPFA™ system:
Why It Matters Globally
The InsightPFA trial establishes the first high-quality randomized evidence for nanosecond PFA worldwide. By demonstrating comparable efficacy to RFA while offering distinct safety and efficiency advantages, this study lays the foundation for a new era of ablation therapy in atrial fibrillation.
With continued clinical experience and technological refinement, LotosPFA™ has the potential to reshape global practice by delivering safer, faster, and more patient-friendly treatment options.